You probably have indigo dyed garments in your wardrobe - over one billion pairs of jeans around the world are dyed blue with indigo.
I recently participated in an indigo dyeing workshop and it was fascinating and fun!
Our instructor was Joann Condino, of Three Pines Studio and she was a fabulous teacher - I know I'm considering future projects.
Indigofera tinctoria was originally domesticated in India, where it is mentioned in manuscripts dating from the 4th century BC. It was recognised as a valuable blue dye by most early explorers of that region. The Venetian explorer Marco Polo described in detail the Indian indigo industry and by the 11th century, Arab traders had introduced indigo to the Mediterranean region, where it replaced their native blue dye plant, woad (Isatis tinctoria).
The cultivation of indigo on a large scale started in the 16th century in India and this was documented by European visitors in the 16th and 17th centuries, particularly in the north of India.
The British established commercial cultivation and production of indigo. Initial plantations began in 1777, and by 1788 most of the production of indigo purchased by the East India Company originated from Bengal. The system became deeply exploitative from 1837 when 'planters' were accorded permission to own land.
The East India Company imported massive volumes of Indian indigo in the mid 1600s. Its use in Europe was clearly a threat to native woad growers. Protests led to the ban of indigo in Britain and other European countries. Despite this, European woad plantations and factories rapidly disappeared.
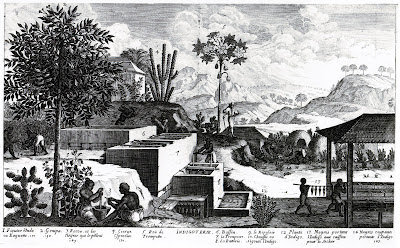
Processing the plant was a cumbersome process:
The cut plant is tied into bundles, which are then packed into the fermenting vats and covered with clear fresh water. The vats, which are usually made of brick lined with cement, have an area of about 400 square feet and are 3 feet deep, are arranged in two rows, the tops of the bottom or "beating vats" being generally on a level with the bottoms of the fermenting vats. The indigo plant is allowed to steep till the rapid fermentation, which quickly sets in, has almost ceased, the time required being from 10-15 hours. The liquor, which varies from a pale straw colour to a golden-yellow, is then run into the beaters, where it is agitated either by men entering the vats and beating with oars, or by machinery. The colour of the liquid becomes green, then blue, and, finally, the indigo separates out as flakes, and is precipitated to the bottom of the vats. The indigo is allowed to thoroughly settle, when the supernatant liquid is drawn off. The pulpy mass of indigo is then boiled with water for some hours to remove impurities, filtered through thick woollen or coarse canvas bags, then pressed to remove as much of the moisture as possible, after which it is cut into cubes and finally air-dried.
But in 1865 the German chemist Johann Friedrich Wilhelm Adolf von Baeyer began working with indigo.
His work culminated in the first synthesis of indigo in 1880 from o-nitrobenzaldehyde and acetone upon addition of dilute sodium hydroxide, barium hydroxide, or ammonia and the announcement of its chemical structure three years later.
BASF developed a commercially feasible manufacturing process that was in use by 1897, and by 1913 natural indigo had been almost entirely replaced by synthetic indigo. In 2002, 17,000 tons of synthetic indigo were produced worldwide.
Here's our dye vat, a five gallon bucket; you can see the "flower" floating on top, a result of oxygen reacting with the dye:
And it comes out green, not turning blue until the dye oxidizes when exposed to oxygen and reverts to its insoluble form.
That's my piece in the center of the clothesline:
And here's a much larger scale piece, created with rocks:
It was amazing how quickly and easily all of us were able to create beautiful and intricate patterns and, of course, I'm now contemplating how I can incorporate indigo into future projects, both for modern and living history purposes - more to come, I'm sure!
Indigofera tinctoria was originally domesticated in India, where it is mentioned in manuscripts dating from the 4th century BC. It was recognised as a valuable blue dye by most early explorers of that region. The Venetian explorer Marco Polo described in detail the Indian indigo industry and by the 11th century, Arab traders had introduced indigo to the Mediterranean region, where it replaced their native blue dye plant, woad (Isatis tinctoria).
The cultivation of indigo on a large scale started in the 16th century in India and this was documented by European visitors in the 16th and 17th centuries, particularly in the north of India.
The British established commercial cultivation and production of indigo. Initial plantations began in 1777, and by 1788 most of the production of indigo purchased by the East India Company originated from Bengal. The system became deeply exploitative from 1837 when 'planters' were accorded permission to own land.
The East India Company imported massive volumes of Indian indigo in the mid 1600s. Its use in Europe was clearly a threat to native woad growers. Protests led to the ban of indigo in Britain and other European countries. Despite this, European woad plantations and factories rapidly disappeared.
Processing the plant was a cumbersome process:
The cut plant is tied into bundles, which are then packed into the fermenting vats and covered with clear fresh water. The vats, which are usually made of brick lined with cement, have an area of about 400 square feet and are 3 feet deep, are arranged in two rows, the tops of the bottom or "beating vats" being generally on a level with the bottoms of the fermenting vats. The indigo plant is allowed to steep till the rapid fermentation, which quickly sets in, has almost ceased, the time required being from 10-15 hours. The liquor, which varies from a pale straw colour to a golden-yellow, is then run into the beaters, where it is agitated either by men entering the vats and beating with oars, or by machinery. The colour of the liquid becomes green, then blue, and, finally, the indigo separates out as flakes, and is precipitated to the bottom of the vats. The indigo is allowed to thoroughly settle, when the supernatant liquid is drawn off. The pulpy mass of indigo is then boiled with water for some hours to remove impurities, filtered through thick woollen or coarse canvas bags, then pressed to remove as much of the moisture as possible, after which it is cut into cubes and finally air-dried.
But in 1865 the German chemist Johann Friedrich Wilhelm Adolf von Baeyer began working with indigo.
His work culminated in the first synthesis of indigo in 1880 from o-nitrobenzaldehyde and acetone upon addition of dilute sodium hydroxide, barium hydroxide, or ammonia and the announcement of its chemical structure three years later.
BASF developed a commercially feasible manufacturing process that was in use by 1897, and by 1913 natural indigo had been almost entirely replaced by synthetic indigo. In 2002, 17,000 tons of synthetic indigo were produced worldwide.
Dyeing with indigo is unique compared to other dyes. Natural indigo takes a considerable amount of effort to get it into a working dye bath because it is insoluble in water. It must go through a process where it is ‘reduced’ and put into a liquid state with the oxygen removed. Although recipes for dye vats vary, all are based on reducing the indigo into a watersoluble form.
However, Jacquard Products have come up with a clever way to make using natural indigo easy: Jacquard’s Indigo is pre-reduced by 60 % and easily mixes with water and therefore makes setting up an indigo vat practically effortless.
In class, we created resist patterns using a variety of methods - rubber bands, clothes pins, rocks, marbles, etc. on large squares of 100% cotton.
Here's our dye vat, a five gallon bucket; you can see the "flower" floating on top, a result of oxygen reacting with the dye:
The fabric needs to be wet when it goes in the vat
And it comes out green, not turning blue until the dye oxidizes when exposed to oxygen and reverts to its insoluble form.
That's my piece in the center of the clothesline:
And here's a much larger scale piece, created with rocks:
It was amazing how quickly and easily all of us were able to create beautiful and intricate patterns and, of course, I'm now contemplating how I can incorporate indigo into future projects, both for modern and living history purposes - more to come, I'm sure!










No comments:
Post a Comment